JAPANESE / ENGLISH

JAPANESE / ENGLISH
Last updated on 05/7/2021
LED is an abbreviation of Light Emission Diode, and is a device which emits light by flowing a current to the p-n junction like a semiconductor laser (LD). It emits various wavelength lights in the ultraviolet, visible and infrared regions, corresponding to its band gap energy. In particular, white LEDs offer long-life and low energy consumption compared with incandescent light bulbs and fluorescent lamps, and thus, are now increasingly used for lighting. White LEDs are also used for many applications in the areas of lighting and display, including LCD backlights used for cell phones, traffic lights, road signs, outdoor displays, and flashlights.
Figure 1 shows the principle of LED light-emitting. The active layer sandwiched between the p- and n-type semiconductors is formed on a sapphire substrate, and voltage is applied across the p-n junction from the electrodes. When forward voltage is applied, electrons conbine with holes at the p-n junction and disappear. At this time, an electron transits from higher energy state to lower state and the excess energy is released as a light.
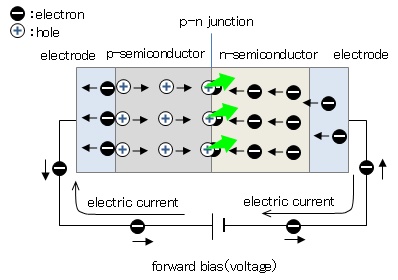
Figure 1: Principle of LED emission.
Figure 2 explains why emission wavelengths are different among semiconductor materials. Combining of holes and electrons at the p-n junction means that the electrons fall from the conduction band to the valence band. When the energy difference between the both bands is greater, a higher-energy light, i.e. a shorter wavelength light is emitted. Since the energy difference(band gap) depends on the semiconductor material, the LED materials are selected on the basis of the band gap to meet the desired light color.
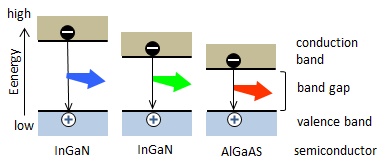
Figure 2: The difference in the emission color by the semiconductor material.(Both of blue and green light emitted from InGaN, but the In/Ga ratio differs.)
There are three ways to obtain white light from LEDs as shown in Fig. 3. The first is the way to irradiate a yellow phosphor by a blue LED. Since yellow is complementary color of blue, the mixture of the blue and yellow looks white. Though this method is most popular because of easy fabrication and high intensity, there is a weak point in the slightly bluish color. The second is the way to emit blue, green, and red phosphor by irradiation of an ultraviolet LED. While the light looks natural and pure white, the light intensity is not as strong as the above method yet. The third is the way to use three LEDs of blue, green, and red. Because the light is intense and any color can be produced, this method is applied to a large display and an LED screen.
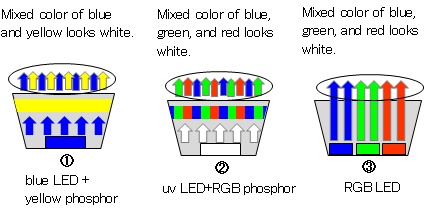
Figure 3: Mechanism of white LED light emitting.
The LED is a semiconductor light-emitting device similar to the LD. What is the difference between the two? Figure 4 shows the basic structures of the LED and the LD. Though the principle of radiation is the same, electrons and holes join at the p-n junction and light is emitted, the properties of the emitted light are different. Since the LED light has random phases, it spreads similarly to light bulbs. On the other hand, the LD light has a specific phase, and therefore it advance straightly without spreading. This difference is attributable to presence or absense of a resonator. The LD can align a phase of a light by a resonator, but the LED without a resonator outputs the light intactly. Another difference is optical fiber coupling losses. The LD light can be launched into an optical fiber with low coupling losses, because the light is outputted from a narrow active layer. Meanwhile, the LED light not be only slightly incident on a fiber, because the LED has a large area of emission.
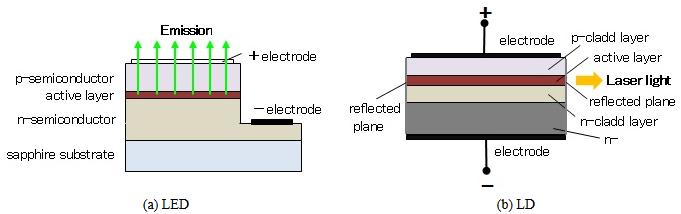
Figure 4: Basic structures of LED and LD.